100 assays per kit
Prices exclude VAT
Available for shipping
| Content: | 100 assays per kit |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | Fructan |
| Assay Format: | Spectrophotometer |
| Detection Method: | Absorbance |
| Wavelength (nm): | 410 |
| Signal Response: | Increase |
| Linear Range: | 2.3 to 55 µg of D-fructose or D-glucose per assay |
| Limit of Detection: | 0.16 g/100 g |
| Total Assay Time: | ~ 90 min |
| Application examples: | Flours, infant formula, animal feed, pet foods, plant materials (e.g. onion), food products and other materials |
| Method recognition: | AACC Method 32-32.01, AOAC Method 999.03, AOAC Method 2016.14, AOAC Method 2018.07 and CODEX Method Type III |
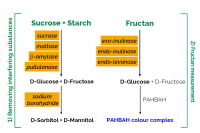
The Fructan Assay Kit is suitable for the specific measurement of fructan in plant extracts, animal feed and food products containing starch, sucrose and other sugars. It is used in three validated methods for the determination of fructan: AOAC method 999.03 (foods), AOAC method 2018.07 (Animal Feed) and AOAC method 2016.14 (infant formula and adult nutritionals).
New, improved procedure.
In the most recent development, a recombinant endo-levanase has been incorporated into the fructanase mixture, extending the use of the method to the measurement of levan-type fructans as are present in grasses such as timothy, cocksfoot, ryegrass and red fescue.
The method described in this booklet employs ultra-pure, recombinant enzymes and specifically measures fructans including inulin-type fructans from chicory, dahlia, jerusalem artichoke; highly branched fructans from onion and wheat stems and leaves; and levan-type fructans from pasture grasses such as timothy grass. The enzymes employed are completely devoid of contaminating enzymes active on β-glucan or gluco-oligosaccharides.
Browse our full range of polysaccharide assay kits.

- Very cost effective
- All kit reagents stable for > 2 years after preparation
- Unaffected by high sucrose / reducing sugar concentrations
- Fructan kits are only available from Megazyme
- Simple format
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
Determination of Fructan (Inulin, FOS, Levan, and Branched Fructan) in Animal Food (Animal Feed, Pet Food, and Ingredients): Single-Laboratory Validation, First Action 2018.07.
McCleary, B. V., Charmier, L. M. J., McKie, V. A., Ciara McLoughlin, C. & Rogowski, A. (2019). Journal of AOAC International, 102(3), 2019 883.
Traditional enzyme-based methods for measurement of fructan were designed to measure just inulin and branched-type (agave) fructans. The enzymes employed, namely exo-inulinase and endo-inulinase, give incompletely hydrolysis of levan. Levan hydrolysis requires a third enzyme, endo-levanase. This paper describes a method and commercial test kit (Megazyme Fructan Assay Kit) for the determination of all types of fructan (inulin, levan, and branched) in a variety of animal feeds and pet foods. The method has been validated in a single laboratory for analysis of pure inulin, agave fructan, levan, and a range of fructan containing samples. Quantification is based on complete hydrolysis of fructan to fructose and glucose by a mixture of exo-inulinase, endo-inulinase, and endo-levanase, followed by measurement of these sugars using the PAHBAH reducing sugar method which gives the same color response with fructose and glucose. Before hydrolysis of fructan, interfering sucrose and starch in the sample are specifically hydrolyzed and removed by borohydride reduction. The single-laboratory validation (SLV) outlined in this document was performed on commercially available inulin (Raftiline) and agave fructan (Frutafit©), levan purified from Timothy grass, two grass samples, a sample of legume hay, two animal feeds and two barley flours, one of which (Barley MAX©) was genetically enriched in fructan through plant breeding. Parameters examined during the validation included working range, target selectivity, recovery, LOD, LOQ, trueness (bias), precision (repeatability and intermediate precision), robustness, and stability. The method is robust, quick, and simple.
Hide AbstractMcCleary, B. V., Charnock, S. J., Rossiter, P. C., O’Shea, M. F., Power, A. M. & Lloyd, R. M. (2006). Journal of the Science of Food and Agriculture, 86(11), 1648-1661.
Procedures for the measurement of starch, starch damage (gelatinised starch), resistant starch and the amylose/amylopectin content of starch, β-glucan, fructan, glucomannan and galactosyl-sucrose oligosaccharides (raffinose, stachyose and verbascose) in plant material, animal feeds and foods are described. Most of these methods have been successfully subjected to interlaboratory evaluation. All methods are based on the use of enzymes either purified by conventional chromatography or produced using molecular biology techniques. Such methods allow specific, accurate and reliable quantification of a particular component. Problems in calculating the actual weight of galactosyl-sucrose oligosaccharides in test samples are discussed in detail.
Hide AbstractMeasurement of total fructan in foods by enzymatic/spectrophotometric method: Collaborative study.
McCleary, B. V., Murphy, A. & Mugford, D. C. (2000). Journal of AOAC International, 83(2), 356-364.
An AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measuring oligofructans and fructan polysaccharide (inulins) in mixed materials and food products. The sample is extracted with hot water, and an aliquot is treated with a mixture of sucrase (a specific sucrose-degrading enzyme), α-amylase, pullulanase, and maltase to hydrolyze sucrose to glucose and fructose, and starch to glucose. These reducing sugars are then reduced to sugar alcohols by treatment with alkaline borohydride solution. The solution is neutralized, and excess borohydride is removed with dilute acetic acid. The fructan is hydrolyzed to fructose and glucose using a mixture of purified exo- and endo-inulinanases (fructanase mixture). The reducing sugars produced (fructose and glucose) are measured with a spectrophotometer after reaction with para-hydroxybenzoic acid hydrazide. The samples analyzed included pure fructan, chocolate, low-fat spread, milk powder, vitamin tablets, onion powder, Jerusalem artichoke flour, wheat stalks, and a sucrose/cellulose control flour. Repeatability relative standard deviations ranged from 2.3 to 7.3%; reproducibility relative standard deviations ranged from 5.0 to 10.8%.
Hide AbstractMcCleary, B. V., Gibson, T. S. & Mugford, D. C. (1997). Journal of AOAC International, 80, 571-579.
An American Association of Cereal Chemists/AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measurement of total starch in a range of cereal grains and products. The flour sample is incubated at 95 degrees C with thermostable alpha-amylase to catalyze the hydrolysis of starch to maltodextrins, the pH of the slurry is adjusted, and the slurry is treated with a highly purified amyloglucosidase to quantitatively hydrolyze the dextrins to glucose. Glucose is measured with glucose oxidase-peroxidase reagent. Thirty-two collaborators were sent 16 homogeneous test samples as 8 blind duplicates. These samples included chicken feed pellets, white bread, green peas, high-amylose maize starch, white wheat flour, wheat starch, oat bran, and spaghetti. All samples were analyzed by the standard procedure as detailed above; 4 samples (high-amylose maize starch and wheat starch) were also analyzed by a method that requires the samples to be cooked first in dimethyl sulfoxide (DMSO). Relative standard deviations for repeatability (RSD(r)) ranged from 2.1 to 3.9%, and relative standard deviations for reproducibility (RSD(R)) ranged from 2.9 to 5.7%. The RSD(R) value for high amylose maize starch analyzed by the standard (non-DMSO) procedure was 5.7%; the value was reduced to 2.9% when the DMSO procedure was used, and the determined starch values increased from 86.9 to 97.2%.
Hide AbstractIdentification of inulin-responsive bacteria in the gut microbiota via multi-modal activity-based sorting.
Riva, A., Rasoulimehrabani, H., Cruz-Rubio, J. M., Schnorr, S. L., von Baeckmann, C., Inan, D., et al. (2023). Nature Communications, 14(1), 8210.
Prebiotics are defined as non-digestible dietary components that promote the growth of beneficial gut microorganisms. In many cases, however, this capability is not systematically evaluated. Here, we develop a methodology for determining prebiotic-responsive bacteria using the popular dietary supplement inulin. We first identify microbes with a capacity to bind inulin using mesoporous silica nanoparticles functionalized with inulin. 16S rRNA gene amplicon sequencing of sorted cells revealed that the ability to bind inulin was widespread in the microbiota. We further evaluate which taxa are metabolically stimulated by inulin and find that diverse taxa from the phyla Firmicutes and Actinobacteria respond to inulin, and several isolates of these taxa can degrade inulin. Incubation with another prebiotic, xylooligosaccharides (XOS), in contrast, shows a more robust bifidogenic effect. Interestingly, the Coriobacteriia Eggerthella lenta and Gordonibacter urolithinfaciens are indirectly stimulated by the inulin degradation process, expanding our knowledge of inulin-responsive bacteria.
Hide AbstractAgave amica a potential model for the study of agavins metabolism.
Salomé-Abarca, L. F., Márquez-López, R. E. & López, M. G. (2023). Scientific Reports, 13(1), 19888.
Fructans found in agave are called agavins, highly branched neo-fructans. They are essential on the yield and quality of Tequila production. The need for agave specimens with higher accumulation of agavins became essential before the growing demand of such products. To get such specimens, understanding agavins metabolism is a quintessential requirement. For this, a more efficient biological model is required. The recently reclassified Agave amica possesses the potential to gather the requirements for becoming such a model. Therefore, this study dealt with the characterization of carbohydrates in the bulbs of A. amica focusing on fructans. Moreover, it tested and described its feasibility as model for the accelerated study of agavins. Infrared analysis unveiled potential content of fructans in the bulbs of A. amica. Furthermore, high performance thin layer chromatography detected fructooligosaccharides. High performance anion exchange chromatography confirmed a polydisperse mixture of branched fructans. Gas chromatography–mass spectrometry analysis demonstrated agavins like structures in the bulbs of A. amica. Moreover, total fructan content and multivariate data analysis through bulb’s age demonstrated their correlation. Thus, the presence of agavins, their correlation with phenology, and their technical advantages highlighted the feasibility of this species as a potential new biological model for the study of agavins’ metabolism.
Hide AbstractTrash or treasure: Rhizome conservation during drought.
Lubbe, F. C., Bitomský, M., Bartoš, M., Marešová, I., Martínková, J. & Klimešová, J. (2023). Functional Ecology, In Press
The role of storage carbohydrates in plant carbon economy is currently disputed as possibly passive accumulation when other resources are limiting growth, or part of a conservative growth strategy as insurance for regrowth and stress response. One indication may be the fate of carbohydrates in senescing rhizomes, as either translocated to be retained in the live and growing end of the rhizome or kept within the senescing rhizome end and lost into the soil for it to decompose. To examine carbohydrate storage in senescing rhizomes, eight rhizomatous species were grown in a split-pot design with one compartment containing the forward-growing and younger end of the rhizome and another containing the older end. Both compartments were either watered (control) or the older one was left un-watered (drought treatment) to trigger rhizome senescence and potential carbohydrate translocation. Plant growth, root traits, and non-structural carbohydrate types and concentrations were assessed in four sequential harvests. Drought treatment plants had higher rhizome dry matter content. Younger rhizome parts produced higher new rhizome and above-ground biomass than older rhizome parts. Carbohydrate concentrations in rhizomes remained consistent for both treatments, younger and older rhizome parts, and all harvests, probably because of the translocation of water from the watered to the dry compartment to prevent senescence and rhizome loss. Contrary to expectations, the experimental treatment did not trigger rhizome senescence: plants responded by conserving the rhizome and resources within, rather than by losing their older parts. The invariant composition and concentration of carbohydrates within the rhizome suggest that rhizomes are essential plant organs and the storage carbohydrates they contain are necessary for regrowth after stress.
Hide AbstractHigh fructan barley lines produced by selective breeding may alter β-glucan and amylopectin molecular structure.
Jayarathna, S., Jin, Y., Dotsenko, G., Fei, M., Andersson, M., Andersson, A. A., Sun, C. & Andersson, R. (2023). Carbohydrate Polymers, 316, 121030.
Six cross-bred barley lines developed by a breeding strategy with the target to enhance the fructan synthesis activity and reduce the fructan hydrolysis activity were analyzed together with their parental lines, and a reference line (Gustav) to determine whether the breeding strategy also affected the content and molecular structure of amylopectin and β-glucan. The highest fructan and β-glucan content achieved in the novel barley lines was 8.6 % and 12 %, respectively (12.3-fold and 3.2-fold higher than in Gustav). The lines with low fructan synthesis activity had higher starch content, smaller building blocks in amylopectin, and smaller structural units of β-glucans than the lines with high-fructan synthesis activity. Correlation analysis confirmed that low starch content was associated with high amylose, fructan, and β-glucan content, and larger building blocks in amylopectin.
Hide AbstractElectrosprayed microparticles from inulin and poly (vinyl) alcohol for colon targeted delivery of prebiotics.
Saud, K. T., Xu, J., Wilkanowicz, S., He, Y., Moon, J. J. & Solomon, M. J. (2023). Food Hydrocolloids, 108625.
Recent studies have highlighted the prebiotic effect of inulin through the selective promotion of colon-residing bacteria, modulation of the composition of the gut microbiome, and consequent generation of beneficial effects on gastrointestinal inflammation, diabetes, and cancer. However, as a water-soluble polysaccharide, the prebiotic effect of inulin is limited by low delivery efficiency and short retention time within the colon. In this study, inulin microparticles (MPs) were produced by the electrospray method, and their material properties and bioavailability were evaluated. Inulin was electrosprayed with poly(vinyl) alcohol (PVA) (MW = 89,000-98,000 g/mol) to improve its processability and mucoadhesive properties. MPs produced at PVA:Inulin mass ratio 1:3 were of diameter 0.42 ± 0.46 μm. FTIR and confocal laser scanning microscopy confirmed the presence and colocalization of the PVA and inulin in the particles. MP suspensions exhibited a time dependent viscoelastic rheological response that trended with time toward the response of the inulin suspension. Additionally, MP suspensions exhibited greater viscosity and shear thinning behavior than their individual components and two-component mixtures. The gut retention of inulin in mice was prolonged when delivered in these MP suspensions relative to inulin suspensions and PVA-inulin two-component mixtures. The increased retention is hypothesized to be a result of the effect of PVA on rheological and mucoadhesive properties. The increased retention of inulin leads to improved availability of inulin for gut microbiota which can support applications in drug delivery and foods.
Hide AbstractReduction of FODMAPs and Amylase-Trypsin Inhibitors in Wheat: A review.
Boakye, P. G., Okyere, A. Y. & Annor, G. A. (2023). Food Hydrocolloids for Health, 3, 100117.
Consumption of fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) can promote gut health in individuals with a healthy gastrointestinal tract. However, FODMAPs, as well as amylase-trypsin inhibitors (ATIs), have been identified as potential triggers of intestinal symptoms in irritable bowel syndrome (IBS) and non-celiac wheat sensitivity (NCWS) patients. Wheat is a major staple worldwide, and hence, accounts for a large proportion of the intake of FODMAPs and ATIs. Thus, this paper aims to provide an overview of the strategies utilized in reducing the levels of FODMAPs and ATIs in wheat.
Hide AbstractFecal microbiota composition affects in vitro fermentation of rye, oat, and wheat bread.
Pirkola, L., Dicksved, J., Loponen, J., Marklinder, I. & Andersson, R. (2023). Scientific Reports, 13(1), 99.
Fermentation of dietary fiber by gut microbes produces short-chain fatty acids (SCFA), but fermentation outcomes are affected by dietary fiber source and microbiota composition. The aim of this study was to investigate the effect of two different fecal microbial compositions on in vitro fermentation of a standardized amount of oat, rye, and wheat breads. Two human fecal donors with different microbial community composition were recruited. Bread samples were digested enzymatically. An in vitro fermentation model was used to study SCFA production, dietary fiber degradation, pH, and changes in microbiota. Feces from donor I had high relative abundance of Bacteroides and Escherichia/Shigella, whereas feces from donor II were high in Prevotella and Subdoligranulum. Shifts in microbiota composition were observed during fermentation. SCFA levels were low in the samples with fecal microbiota from donor I after 8 h of fermentation, but after 24 h acetate and propionate levels were similar in the samples from the different donors. Butyrate levels were higher in the fermentation samples from donor II, especially with rye substrate, where high abundance of Subdoligranulum was observed. Dietary fiber degradation was also higher in the fermentation samples from donor II. In conclusion, fermentation capacity and substrate utilization differed between the two different microbiota compositions.
Hide AbstractComposition of Polysaccharides in Hull-Less Barley Sourdough Bread and Their Impact on Physical Properties of Bread.
Reidzane, S., Gramatina, I., Galoburda, R., Komasilovs, V., Zacepins, A., Bljahhina, A., Kince T., Traksmaa, A. & Klava, D. (2022). Foods, 12(1), 155.
The complex of polysaccharides of the grain transforms during processing and modifies the physical and chemical characteristics of bread. The aim of the research was to characterize the changes of glucans, mannans and fructans in hull-less barley and wholegrain wheat breads fermented with spontaneous hull-less barley sourdough, germinated hull-less barley sourdough and yeast, as well as to analyze the impact of polysaccharides on the physical parameters of bread. By using the barley sourdoughs for wholegrain wheat bread dough fermentation, the specific volume and porosity was reduced; the hardness was not significantly increased, but the content of β-glucans was doubled. Principal component analysis indicates a higher content of β-glucans and a lower content of starch, total glucans, fructans and mannans for hull-less barley breads, but wholegrain wheat breads fermented with sourdoughs have a higher amount of starch, total glucans, fructans and mannans, and a lower content of β-glucans. The composition of polysaccharides was affected by the type of flour and fermentation method used.
Hide AbstractAsparagus Fructans as Emerging Prebiotics.
Hamdi, A., Viera-Alcaide, I., Guillén-Bejarano, R., Rodríguez-Arcos, R., Muñoz, M. J., Monje Moreno, J. M. & Jiménez-Araujo, A. (2023). Foods, 12(1), 81.
Commercial fructans (inulin and oligofructose) are generally obtained from crops such as chicory, Jerusalem artichoke or agave. However, there are agricultural by-products, namely asparagus roots, which could be considered potential sources of fructans. In this work, the fructans extracted from asparagus roots and three commercial ones from chicory and agave were studied in order to compare their composition, physicochemical characteristics, and potential health effects. Asparagus fructans had similar chemical composition to the others, especially in moisture, simple sugars and total fructan contents. However, its contents of ash, protein and phenolic compounds were higher. FTIR analysis confirmed these differences in composition. Orafti®GR showed the highest degree of polymerization (DP) of up to 40, with asparagus fructans (up to 25) falling between Orafti®GR and the others (DP 10-11). Although asparagus fructan powder had a lower fructan content and lower DP than Orafti®GR, its viscosity was higher, probably due to the presence of proteins. The existence of phenolic compounds lent antioxidant activity to asparagus fructans. The prebiotic activity in vitro of the four samples was similar and, in preliminary assays, asparagus fructan extract presented health effects related to infertility and diabetes diseases. All these characteristics confer a great potential for asparagus fructans to be included in the prebiotics market.
Hide AbstractEPS-Producing Microorganisms from Louisiana’s Crusher Juice and the Effect of Processing Conditions on EPS Production.
Aita, G. M. & Moon, Y. H. (2022). Sugar Tech, 1-9.
During microbial deterioration of sugarcane, a variety of extracellular polysaccharides (EPS, e.g., dextran, fructans) and other impurities (e.g., organic acids, sugar alcohols, alcohols) are produced. The microbial-derived EPS dextran (a polymer of glucose) has generally been considered the main problem in sugarcane processing, with its main production source being attributed to the bacterium Leuconostoc mesenteroides. Technical problems associated with the presence of dextran at the sugar factory can affect every step of sugar processing resulting in high viscosities, reduced efficiencies, elongation of sugar crystals, and the loss of sucrose to molasses. In recent years, fructans (a polymer of fructose) concentrations have been increasingly reported in both cane juice and molasses at Louisiana sugar factories. With the limited information that exists on the microbial origin of fructans at the sugar factories and its possible impact on Louisiana sugarcane processing, this research study aimed at identifying the microbial populations present in high numbers in the crusher juice or first extraction juice, and determining the effect of processing conditions on their ability to produce EPS. Our findings indicate that Lactobacillus, Lactococcus, Leuconostoc, Pantoea, Pseudomonas, and Saccharomyces were the microbial genera present in high cell numbers and that all isolates were capable of producing dextran and/or fructans. Leuconostoc had the most diverse number of species. A single isolate of L. suionicum A14 was identified and produced only fructans from sucrose. This is the first study to report on a fructan-only producing Leuconostoc isolate from sugarcane. EPS production was affected by temperature, sucrose concentrations, and medium pH.
Hide AbstractSelf-assembly levan, as a natural active bio-nanocarrier.
Lewińska, A., Domżał-Kędzia, M. & Łukaszewicz, M. (2023). Sustainable Chemistry and Pharmacy, 31, 100936.
Levan, a homopolysaccharide, has great potential as a functional biopolymer in foods, feeds, cosmetics, and the pharmaceutical and chemical industries. Two types of nanocarrier of levan obtained from Bacillus subtilis strain, as potential delivery system carriers, are studied in this work. The first type of polymer nanoparticles are created in water by a self-assembly process directly from fermentation, while the second type are created after precipitating a levan powder and dissolving it in water. Both types of the obtained nanoparticles were of a size in the range of 214.10 ± 0.70–238.27 ± 1.72 nm with low polydispersity. The nanoparticle systems were found to be visually and kinetically stable. There were differences in the surface morphology of the carriers: the first type had an irregular surface while the second type were smooth. Differences in their ability to scavenge free radicals were also demonstrated: nanoparticles from culture supernatant scoured 8.57% more radicals than nanoparticles from precipitated polymer. Both types are able to encapsulate hydrophobic compounds and penetrate the stratum corneum. Analysis using Raman spectroscopy showed that the nanoparticles increased hydration in the deeper layers of the skin. The diversity exhibited by the two types of levan nanoparticles would indicate a wide range of potential applications in the cosmetics industry.
Hide Abstract