100 assays (manual) / 1000 assays (microplate) / 1100 assays (auto-analyser)
Prices exclude VAT
Available for shipping
| Content: | 100 assays (manual) / 1000 assays (microplate) / 1100 assays (auto-analyser) |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | Trehalose |
| Assay Format: | Spectrophotometer, Microplate, Auto-analyser |
| Detection Method: | Absorbance |
| Wavelength (nm): | 340 |
| Signal Response: | Increase |
| Linear Range: | 4 to 80 µg of trehalose per assay |
| Limit of Detection: | 37.5 mg/L |
| Reaction Time (min): | ~ 10 min |
| Application examples: | Honey, mushrooms, bread, beer, seafood (e.g. lobster and shrimp), fruit juices, purees and fillings, nutrition bars, surimi, dehydrated fruits and vegetables, fruit products, white chocolate, sports drinks, dairy products, egg products, soups and sauces, confectionery, chewing gum, cosmetics, pharmaceuticals and other materials (e.g. biological cultures, samples, etc.). |
| Method recognition: | Novel method |
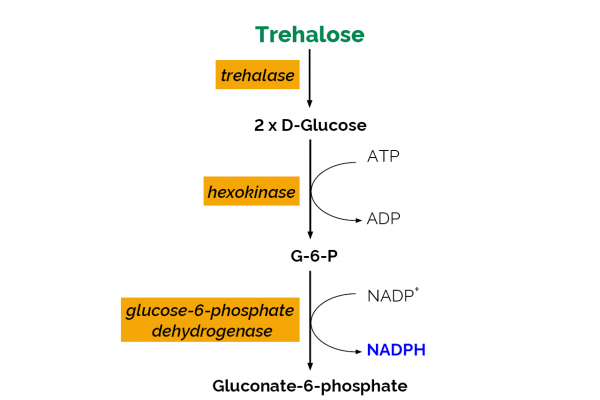
The Trehalose assay kit is a simple method for the rapid and reliable measurement and analysis of trehalose in foods, beverages and other materials.
Note for Content: The number of manual tests per kit can be doubled if all volumes are halved. This can be readily accommodated using the MegaQuantTM Wave Spectrophotometer (D-MQWAVE).
Browse our full range of monosaccharide and disaccharide test kits.

- Only enzymatic kit available
- Very cost effective
- All reagents stable for > 2 years after preparation
- Very rapid reaction
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
- Suitable for manual, microplate and auto-analyser formats
Deletion of NTH1 and HSP12 increases the freeze-thaw resistance of baker’s yeast in bread dough.
Chen, B. C. & Lin, H. Y. (2022). Microbial Cell Factories, 21(1), 1-10.
Background: The intracellular molecule trehalose in Saccharomyces cerevisiae may have a major protective function under extreme environmental conditions. NTH1 is one gene which expresses trehalase to degrade trehalose. Small heat shock protein 12 (HSP12 expressed) plays a role in protecting membranes and enhancing freezing stress tolerance. Results: An optimized S. cerevisiae CRISPR-Cpf1 genome-editing system was constructed. Multiplex genome editing using a single crRNA array was shown to be functional. NTH1 or/and HSP12 knockout in S. cerevisiae enhanced the freezing stress tolerance and improved the leavening ability after freezing and thawing. Conclusions: Deleting NTH1 in the combination with deleting HSP12 would strengthen the freezing tolerance and protect the cell viability from high rates of death in longer-term freezing. It provides valuable insights for breeding novel S. cerevisiae strains for the baking industry through a more precise, speedy, and economic genome-editing system.
Hide AbstractIncreased Stress Levels in Caged Honeybee (Apis mellifera)(Hymenoptera: Apidae) Workers.
Lattorff, H. M. G. (2022). Stresses, 2(4), 373-383.
Honeybees, Apis mellifera, usually live in large colonies consisting of thousands of individuals. Within the colony, workers interact with their social environment frequently. The large workforce, division of labour, and other features may promote the ecological success of honeybees. For decades, artificial mini colonies in cages within the laboratory have become the gold standard, especially in experiments related to toxicology, effects of pesticides and pathogens. Experiments using caged bees and full-sized colonies yielded contradictory results. Here, the effect of cage experiments on the stress level of individual bees is analysed. Two different stress response were targeted, the heat shock response and the mobilization of energetic resources. While no differences were found for varying group sizes of bees, very strong effects emerged by comparing caged workers with bees from natural colonies. Caged workers showed increased levels of hsp expression and reduced haemolymph titres for trehalose, the energy storage sugar. These results reveal that the lack of the social environment (e.g., lack of queen, lack of sufficient group size) induce stress in caged bees, which might act synergistically when bees are challenged by additional stressors (e.g., pesticides, pathogens) resulting in higher mortality than observed under field conditions.
Hide AbstractChronic exposure to field-realistic doses of imidacloprid resulted in biphasic negative effects on honey bee physiology.
Kim, S., Kim, J. H., Cho, S., Clark, J. M. & Lee, S. H. (2022). Insect Biochemistry and Molecular Biology, 144, 103759.
There have been many investigations on the negative effects of imidacloprid (IMD) on honey bees. IMD is known to disrupt honey bee physiology and colony health at a relatively low concentration compared to other pesticides. In this study, honey bee colonies were chronically exposed to field-realistic concentrations (5, 20, and 100 ppb) of IMD, and the body weight, flight performance, carbohydrate reserve, and lipid contents of forager bees analyzed. Transcriptome analyses followed by quantitative PCR were also conducted for both nurse and forager bees to elucidate any changes in energy metabolism related to phenotypic disorders. The body weights of newly emerged and nurse bees showed decreasing tendencies as the IMD concentration increased. In forager bees, however, IMD induced a biphasic change in body weight: body weight was decreased at the lower concentrations (5 and 20 ppb) but increased at the higher concentration (100 ppb). Nevertheless, the flight capability of forager bees significantly decreased in a concentration-dependent manner. The effects of IMD on target gene transcription in forager bees showed biphasic patterns between low (5 and 20 ppb) and high (100 ppb) concentrations. Nurse bees showed typical features of premature transition to foragers in a concentration-dependent manner. When exposed to low concentrations, forager bees exhibited downregulation of genes involved in carbohydrate and lipid metabolism and in the insulin/insulin-like growth factor signaling pathway, upregulation of transporter activity, and a dose-dependent body weight reduction, which were similar to insulin resistance and diabetic symptoms. However, increased lipid metabolism and decreased energy metabolism with body weight gain were observed at high IMD concentration. Considered together, these results suggest that field-realistic doses of IMD alter honey bee energy metabolism in distinctly different ways at low and high concentrations, both of which negatively affect honey bee colony health.
Hide AbstractEffects of Water Stress on Quality and Sugar Metabolism in ‘Gala’Apple Fruit.
Tao, H., Sun, H., Wang, Y., Wang, X. & Guo, Y. (2022). Horticultural Plant Journal, In Press.
Sugar plays an important role in apple fruit development, appearance and quality as well as contributing to a plant's water stress response. Trehalose and the trehalose biosynthetic metabolic pathways are part of the sugar signaling system in plants, which are important regulator of water stress response in apple. The effect of water stress treatments applied to apple trees and the corresponding effects of ABA on developmental fruit quality were examined for indicators of fruit quality during fruit development. The results indicated that the severe water stress treatment (W2) occurring after the last stage of fruit cell division caused a decrease in the color and size of fruit. The moderate water stress (W1) occurring after the last stage of fruit cell enlargement (S2) caused an increase in the content of fructose and sorbitol while the apple fruit shape was not affected. These changes in sugar are related to the activity of sugar metabolic enzymes. While the enzymatic activity of vacuolar acid invertase (vAINV) was higher, that of sucrose-phosphate synthase (SPS) was lower in water stress treated fruit throughout the developmental period. This indicates that enhanced sucrose degradation and reduced sucrose synthesis leads to an overall reduced sucrose content during times of drought. Thus, water stress reduced sucrose content. Whereas the content of endogenous trehalose and ABA were the highest in water stress treated fruit. A moderate water stress (W1) imposed on apple trees via water restriction (60%–65% of field capacity) after the fruit cell enlargement phase of fruit development yielded sweeter fruit of higher economic value.
Hide AbstractTrehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions.
Santos, J. L. & Ebert, D. (2022). Biology Letters, 18(2), 20210615.
Environmental fluctuations often select for adaptations such as diapause states, allowing species to outlive harsh conditions. The natural sugar trehalose which provides both cryo- and desiccation-protection, has been found in diapause stages of diverse taxa. Here, we hypothesize that trehalose deposition in resting stages is a locally adapted trait, with higher concentrations produced in harsher habitats. We used resting stages, produced under standardized conditions, by 37 genotypes of Daphnia magna collected from Western Palaearctic habitats varying in their propensity to dry in summer and freeze in winter. Resting eggs produced by D. magna from populations from summer-dry habitats showed significantly higher trehalose than those from summer-wet habitats, suggesting that trehalose has a protective function during desiccation. By contrast, winter-freezing did not explain variation in trehalose content. Adaptations to droughts are important, as summer dryness of water bodies is foreseen to increase with ongoing climate change.
Hide AbstractThe photosynthetic efficiency and carbohydrates responses of six edamame (Glycine max. L. Merrill) cultivars under drought stress.
Hlahla, J. M., Mafa, M. S., Van der Merwe, R., Alexander, O., Duvenhage, M. M., Kemp, G. & Moloi, M. J. (2022). Plants, 11(3), 394.
Vegetable-type soybean, also known as edamame, was recently introduced to South Africa. However, there is lack of information on its responses to drought. The aim of this study was to investigate the photosynthetic efficiency and carbohydrates responses of six edamame cultivars under drought stress. Photosynthetic efficiency parameters, including chlorophyll fluorescence and stomatal conductance, were determined using non-invasive methods, while pigments were quantified spectrophotometrically. Non-structural carbohydrates were quantified using Megazyme kits. Structural carbohydrates were determined using Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). Drought stress significantly increased the Fv/Fm and PIabs of AGS429 and UVE17 at pod filling stage. Chlorophyll-a, which was most sensitive to drought, was significantly reduced in AGS429 and UVE17, but chlorophyll-b was relatively stable in all cultivars, except UVE17, which showed a significant decline at flowering stage. AGS354 and AGS429 also showed reduced chlorophyll-b at pod filling. UVE17 showed a significant reduction in carotenoid content and a substantial reduction in stomatal conductance during pod filling. Drought stress during pod filling resulted in a significant increase in the contents of trehalose, sucrose and starch, but glucose was decreased. Chlorophyll-a positively correlated with starch. The FTIR and XRD results suggest that the cell wall of UVE14, followed by UVE8 and AGS429, was the most intact during drought stress. It was concluded that carotenoids, stomatal conductance, starch and hemicellulose could be used as physiological/biochemical indicators of drought tolerance in edamame. This information expands our knowledge of the drought defense responses in edamame, and it is essential for the physiological and biochemical screening of drought tolerance.
Hide AbstractRoles of PINK1 in regulation of systemic growth inhibition induced by mutations of PTEN in Drosophila.
Han, Y., Zhuang, N., & Wang, T. (2021). Cell Reports, 34(12), 108875.
The maintenance of mitochondrial homeostasis requires PTEN-induced kinase 1 (PINK1)-dependent mitophagy, and mutations in PINK1 are associated with Parkinson’s disease (PD). PINK1 is also downregulated in tumor cells with PTEN mutations. However, there is limited information concerning the role of PINK1 in tissue growth and tumorigenesis. Here, we show that the loss of pink1 caused multiple growth defects independent of its pathological target, Parkin. Moreover, knocking down pink1 in muscle cells induced hyperglycemia and limited systemic organismal growth by the induction of Imaginal morphogenesis protein-Late 2 (ImpL2). Similarly, disrupting PTEN activity in multiple tissues impaired systemic growth by reducing pink1 expression, resembling wasting-like syndrome in cancer patients. Furthermore, the re-expression of PINK1 fully rescued defects in carbohydrate metabolism and systemic growth induced by the tissue-specific pten mutations. Our data suggest a function for PINK1 in regulating systemic growth in Drosophila and shed light on its role in wasting in the context of PTEN mutations.
Hide AbstractSulfolobus acidocaldarius uses a complex trehalose metabolism for salt stress response involving a novel TPS/TPP pathway.
Stracke, C., Meyer, B. H., Hagemann, A., Jo, E., Lee, A., Albers, S. V., Cha, J., Brasen, C. & Siebers, B. (2020). Applied and Environmental Microbiology, 86(24).
The crenarchaeon Sulfolobus acidocaldarius has been described to synthesize trehalose via the maltooligosyltrehalose synthase (TreY) and maltooligosyltrehalose trehalohydrolase (TreZ) pathway, and the trehalose glycosyltransferring synthase (TreT) pathway has been predicted. Deletion mutant analysis of strains with single and double deletions of ΔtreY and ΔtreT in S. acidocaldarius revealed that in addition to these two pathways, a third, novel trehalose biosynthesis pathway is operative in vivo: the trehalose-6-phosphate (T6P) synthase/T6P phosphatase (TPS/TPP) pathway. In contrast to known TPS proteins, which belong to the GT20 family, the S. acidocaldarius TPS belongs to the GT4 family, establishing a new function within this group of enzymes. This novel GT4-like TPS was found to be present mainly in the Sulfolobales. The ΔtreY ΔtreT Δtps triple mutant of S. acidocaldarius, which lacks the ability to synthesize trehalose, showed no altered phenotype under standard conditions or heat stress but was unable to grow under salt stress. Accordingly, in the wild-type strain, a significant increase of intracellular trehalose formation was observed under salt stress. Quantitative real-time PCR showed a salt stress-mediated induction of all three trehalose-synthesizing pathways. This demonstrates that in Archaea, trehalose plays an essential role for growth under high-salt conditions.
Hide AbstractEngineering cyanobacteria as cell factories for direct trehalose production from CO2.
Qiao, Y., Wang, W. & Lu, X. (2020). Metabolic Engineering, 62, 161-171.
Trehalose is a non-reducing disaccharide with a wide range of applications in food, cosmetic, and pharmaceutical industries. Cyanobacteria are promising cell factories to produce biochemicals by using solar energy and CO2. Trehalose is biosynthesized at low intracellular concentrations as a salt-inducible compatible solute in some cyanobacteria. In the current study, we demonstrated the efficient trehalose production without salt induction in cyanobacteria by metabolic engineering. The trehalose transporter 1 (TRET1) from an anhydrobiotic insect (Polypedilum vanderplanki) was successfully expressed in the engineered strains and the intracellular trehalose was efficiently secreted to the medium. As the results, the engineered strain co-expressing maltooligosyl trehalose synthase (MTS), maltooligosyl trehalose trehalohydrolase (MTH) and TRET1 secreted 97% of trehalose to the medium, and the titer was up to 2.7 g/L in 15 days. In addition, 5.7 g/L trehalose was produced by semi-continuous cultivation in 34 days. Taken together, this work demonstrates cyanobacteria can be applied as cell factories for direct sunlight-driven conversion of CO2 into excreted trehalose.
Hide AbstractThe effect of trehalose on autophagy-related proteins and cyst growth in a hypomorphic Pkd1 mouse model of autosomal dominant polycystic kidney disease.
Atwood, D. J., Brown, C. N., Holditch, S. J., Pokhrel, D., Thorburn, A., Hopp, K. & Edelstein, C. L. (2020). Cellular Signalling, 75, 109760.
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited disorder characterized by kidney cyst growth often resulting in end-stage renal disease. There is growing attention on understanding the role of impaired autophagy in ADPKD. Trehalose (TRE) has been shown to increase both protein stability and aggregate clearance and induce autophagy in neurodegenerative diseases. TRE treatment in wild type mice compared to vehicle resulted in increased expression in the kidney of Atg12-5 complex and increased Rab9a, autophagy-related proteins that play a role in the formation of autophagosomes. Thus, the aim of the study was to determine the effect of TRE on cyst growth and autophagy-related proteins, in the hypomorphic Pkd1RC/RC mouse model of ADPKD. Pkd1RC/RC mice were treated 2% TRE in water from days 50 to 120 of age. TRE did not slow cyst growth or improve kidney function or affect proliferation and apoptosis in Pkd1RC/RC kidneys. In Pkd1RC/RC vs. wild type kidneys, expression of the Atg12-5 complex was inhibited by TRE resulting in increased free Atg12 and TRE was unable to rescue the deficiency of the Atg12-5 complex. Rab9a was decreased in Pkd1RC/RC vs. wild type kidneys and unaffected by TRE. The TRE-induced increase in p62, a marker of autophagic cargo, that was seen in normal kidneys was blocked in Pkd1RC/RC kidneys. In summary, the autophagy phenotype in Pkd1RC/RC kidneys was characterized by decreases in crucial autophagy-related proteins (Atg12-5 complex, Atg5, Atg16L1), decreased Rab9a and increased mTORC1 (pS6S240/244, pmTORS2448) proteins. TRE increased Atg12-5 complex, Rab9a and p62 in normal kidneys, but was unable to rescue the deficiency in autophagy proteins or suppress mTORC1 in Pkd1RC/RC kidneys and did not protect against cyst growth.
Hide AbstractCombination of hydrophobically modified γ-poly (glutamic acid) and trehalose achieving high cryosurvival of RBCs.
Zhang, Q., Liu, B., Chong, J., Ren, L., Zhao, Y. & Yuan, X. (2020). Science China Technological Sciences, 1-11.
Trehalose is expected to be an alternative for toxic glycerol as a biocompatible cryoprotectant of red blood cells (RBCs). In this work, γ-poly(glutamic acid) (PGA) is modified by grafting hydrophobic phenethylamine, 3,4-dimethoxyphenylethylamine and dodecylamine, respectively. The graft-modified PGA can significantly enhance cryosurvival of RBCs in combination with trehalose. Analyses of dynamic light scattering, hemolysis assay, atomic force microscope and confocal laser scanning microscope suggest that the modified PGA polymers can self-assemble into nanoparticles in phosphate buffer saline solutions at the pH range of 6.0-7.4, and exhibit membrane-disruptive activity due to hydrogen bond, conjugation and hydrophobic interactions with cell membranes. It is assumed that the modified PGA polymers can improve the cryosurvival of RBCs by promoting membrane permeability of trehalose. Among the three graft-modified polymers, phenethylamine-grafted PGA (PGA-g-PEA) can significantly increase the intracellular trehalose-loading content to 11.3±2.4 mM at pH 7.4, much higher than the value 0.17±0.66 mM when trehalose is used without any polymers. In view of the aforementioned merit, the cryosurvival rate of sheep RBCs is increased to about 90% by incubation with 1.0 mg mL-1 PGA-g-PEA and 0.36 M trehalose. In vitro cell culture of L929 fibroblasts demonstrates low cytotoxicity of PGA-g-PEA. Therefore, hydrophobic PEA-modified PGA with enhanced intracellular trehalose-loading ability can be potentially applied in glycerol-free RBC cryopreservation or other related biomacromolecule delivery systems.
Hide AbstractSequencing and analysis of the genome of propionibacterium freudenreichii T82 strain: importance for industry.
Piwowarek, K., Lipińska, E., Hać-Szymańczuk, E., Kieliszek, M. & Kot, A. M. (2020). Biomolecules, 10(2), 348.
The genome of Propionibacterium freudenreichii ssp. Freudenreichii T82, which has a chromosome containing 2,585,340 nucleotides with 67.3% GC content (guanine-cytosine content), is described in this paper. The total number of genes is 2308, of which 2260 are protein-coding genes and 48 are RNA genes. According to the genome analysis and the obtained results, the T82 strain can produce various compounds such as propionic acid, trehalose, glycogen, and B group vitamins (e.g., B6, B9, and B12). From protein-coding sequences (CDSs), genes related to stress adaptation, biosynthesis, metabolism, transport, secretion, and defense machinery were detected. In the genome of the T82 strain, sequences corresponding to the CRISPR loci (Clustered Regularly Interspaced Short Palindromic Repeats), antibiotic resistance, and restriction–modification system were found.
Hide AbstractUnusual deprivation of compatible solutes in Acinetobacter baumannii.
Zeidler, S. & Müller, V. (2020). Environmental Microbiology, 22(4), 1370-1380.
The opportunistic human pathogen Acinetobacter baumannii is one of the leading causes of nosocomial infections. The high prevalence of multidrug‐resistant strains, a high adaptability to changing environments and an overall pronounced stress resistance contribute to persistence and spread of the bacteria in hospitals and thereby promote repeated outbreaks. Altogether, the success of A. baumannii is mainly built on adaptation and stress resistance mechanisms, rather than relying on ‘true’ virulence factors. One of the stress factors that pathogens must cope with is osmolarity, which can differ between the external environment and different body parts of the human host. A. baumannii ATCC 19606T accumulates the compatible solutes glutamate, mannitol and trehalose in response to high salinities. In this work, it was found that most of the solutes vanish immediately after reaching stationary phase, a very unusual phenomenon. While glutamate can be metabolized, mannitol produced by MtlD is excreted to the medium in high amounts. First results indicate that A. baumannii ATCC 19606T undergoes a rapid switch to a dormant state (viable but non‐culturable) after disappearance of the compatible solutes. Resuscitation from this state could easily be achieved in PBS or fresh medium.
Hide AbstractCanola cultivars affect nutrition and cold hardiness of Plutella xylostella (L.)(Lepidoptera: Plutellidae).
Nouri-Ganbalani, G., Naseri, B., Majd-Marani, S. & Borzoui, E. (2020). International Journal of Tropical Insect Science, 1-10.
The diamondback moth, Plutella xylostella (L.), has become the most destructive insect pest of Brassica crop plants, such as B. napus throughout the world including Iran. In this study, nutritional indices, digestive enzyme activity and cold hardiness of P. xylostella on seven canola cultivars including Delgan, H19, Modena, Okapi, Opera, RGS003, and SLM046 were studied under laboratory conditions (25 ± 1°C, 65 ± 5% RH, 16:8 L:D). Fourth instar larvae fed on cultivar SLM046 had the highest efficiency of conversion of ingested and digested food (4.35 ± 0.24% and 4.99 ± 0.31%, respectively). Relative consumption rate (RCR) of P. xylostella 4th instar was higher when fed on Opera cultivar (5.62 ± 0.15 mg/mg/day) while it was lower on Okapi (3.33 ± 0.15 mg/mg/day). The larvae fed on cultivars SLM046 and Okapi had the highest (0.194 ± 0.007 mg/mg/day) and the lowest (0.088 ± 0.003 mg/mg/day) relative growth rate (RGR), respectively. We found a significantly higher amylolytic and proteolytic activity in the midgut of the larvae fed cultivar SLM046. Our findings showed that the activity of enzyme inhibitors and polyphenol oxidase, as antidigestive compounds, are major reasons for the low nutritional efficiency of P. xylostella larvae on some cultivars. The results of the present study indicate that cultivar Okapi is an unsuitable host for the feeding of P. xylostella. These results develop our knowledge of the negative effects of plant defenses on P. xylostella.
Hide AbstractPartitioning sources of CO2 emission after soil wetting using high-resolution observations and minimal models.
Slessarev, E. W. & Schimel, J. P. (2020). Soil Biology and Biochemistry, 143, 107753.
complex, evolving through several stages before subsiding-possibly reflecting contributions from multiple carbon (C) sources. We hypothesized that respiration after wetting combines C from cellular sources (notably trehalose, a cellular osmo-protectant) and soluble extracellular C made available by the physical effects of wetting, with cellular sources contributing early during the pulse and extracellular C dominating later. We tested this hypothesis by making measurements of soil respiration, microbial biomass, trehalose, and water extractable organic C after wetting dry soil in the laboratory. We then devised several alternative minimal models to evaluate the potential contributions of trehalose and extracellular C to the respiration pulse. We found that the chloroform-labile fraction of the microbial biomass was comprised of 50% trehalose in dry soil. Both trehalose and water extractable organic C pools declined rapidly after wetting; trehalose disappeared within 3 h of water addition. Chloroform-labile glucose increased 3 h after water addition and then declined, suggesting rapid hydrolysis of trehalose and consumption of the resulting glucose. Respiration dynamics after wetting could be best explained by models that included two C sources, combining rapid metabolism of trehalose with slower consumption of extracellular soluble C. These results suggest that pulses of respiration after wetting reflect both osmolyte consumption within the microbial biomass and enhanced extracellular C availability; hence, models of microbial drought response must account for both processes to fully resolve soil C dynamics.
Hide Abstract