10 g
Prices exclude VAT
Available for shipping
| Content: | 10 g or 50 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 2 years under recommended storage conditions |
| CAS Number: | 9014-63-5 |
| Source: | Beechwood |
| Purity: | > 95% |
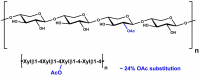
| Monosaccharides (%): | Xylose: Glucuronic Acid: Other sugars = 86.1: 11.3: 2.6 |
| Main Chain Glycosidic Linkage: | β-1,4 and α-1,2 |
| Substrate For (Enzyme): | endo-1,4-β-Xylanase |
Highly purified xylan from beechwood for use in research, biochemical enzyme assays and in vitro diagnostic analysis.
Suitable as a replacement for birchwood xylan as a substrate for β-xylanase in DNSA reducing sugar assay.
Data booklets for each pack size are located in the Documents tab.
Mangan, D., Cornaggia, C., Liadova, A., McCormack, N., Ivory, R., McKie, V. A., Ormerod, A. & McCleary, D. V. (2017). Carbohydrate Research, 445, 14-22.
endo-1,4-β-Xylanase (EC 3.2.1.8) is employed across a broad range of industries including animal feed, brewing, baking, biofuels, detergents and pulp (paper). Despite its importance, a rapid, reliable, reproducible, automatable assay for this enzyme that is based on the use of a chemically defined substrate has not been described to date. Reported herein is a new enzyme coupled assay procedure, termed the XylX6 assay, that employs a novel substrate, namely 4,6-O-(3-ketobutylidene)-4-nitrophenyl-β-45-O-glucosyl-xylopentaoside. The development of the substrate and associated assay is discussed here and the relationship between the activity values obtained with the XylX6 assay versus traditional reducing sugar assays and its specificity and reproducibility were thoroughly investigated.
Hide AbstractMcCleary, B. V. & McGeough, P. (2015). Appl. Biochem. Biotechnol., 177(5), 1152-1163.
The most commonly used method for the measurement of the level of endo-xylanase in commercial enzyme preparations is the 3,5-dinitrosalicylic acid (DNS) reducing sugar method with birchwood xylan as substrate. It is well known that with the DNS method, much higher enzyme activity values are obtained than with the Nelson-Somogyi (NS) reducing sugar method. In this paper, we have compared the DNS and NS reducing sugar assays using a range of xylan-type substrates and accurately compared the molar response factors for xylose and a range of xylo-oligosaccharides. Purified beechwood xylan or wheat arabinoxylan is shown to be a suitable replacement for birchwood xylan which is no longer commercially available, and it is clearly demonstrated that the DNS method grossly overestimates endo-xylanase activity. Unlike the DNS assay, the NS assay gave the equivalent colour response with equimolar amounts of xylose, xylobiose, xylotriose and xylotetraose demonstrating that it accurately measures the quantity of glycosidic bonds cleaved by the endo-xylanase. The authors strongly recommend cessation of the use of the DNS assay for measurement of endo-xylanase due to the fact that the values obtained are grossly overestimated due to secondary reactions in colour development.
Hide AbstractXylooligosaccharides produced from sugarcane leaf arabinoxylan using xylanase from Aureobasidium pullulans NRRL 58523 and its prebiotic activity toward Lactobacillus spp.
Nongkhai, S. N., Piemthongkham, P., Bankeeree, W., Punnapayak, H., Lotrakul, P. & Prasongsuk, S. (2023). Heliyon, 9(11).
In an attempt to enhance the value of sugarcane leaf, xylan was extracted and used for xylooligosaccharide (XO) production via enzymatic hydrolysis using xylanase from the black yeast Aureobasidium pullulans. The xylan was extracted from sugarcane leaf using alkali extraction according to the response surface methodology. The highest xylan yield (99.42 ± 4.05 % recovery) was obtained using 14.32 % (w/v) NaOH, 13.25:1 liquid: solid ratio, at 121°C and 15 lb.in2 for 32 min. Sugar composition and FTIR spectrum analyses confirmed its structure as arabinoxylan. The extracted arabinoxylan had a relatively high molecular weight compared to previous studies. Crude endoxylanase from A. pullulans NRRL 58523 was selected for enzymatic hydrolysis of the xylan. The enzyme hydrolyzed well at 50 °C, pH 4.0 and was relatively stable under this condition (87.38 ± 1.26 % of the activity remained after 60 h). XOs, especially xylobiose and xylotriose, were obtained at the maximum yield of 237.51 ± 17.69 mg/g xylan via endoxylanase hydrolysis under the optimum conditions (50°C, pH 4.0, 65.31 U/g xylan, 53 h). XOs exhibited species-specific prebiotic activity toward three strains of Lactobacillus spp. but not toward Bifidobacterium spp.
Hide AbstractXylobiose treatment triggers a defense-related response and alters cell wall composition.
Dewangan, B. P., Gupta, A., Sah, R. K., Das, S., Kumar, S., Bhattacharjee, S. & Pawar, P. A. M. (2023). Plant Molecular Biology, 113(6), 383-400.
Plant cell wall-derived oligosaccharides, i.e., damage-associated molecular patterns (DAMPs), could be generated after pathogen attack or during normal plant development, perceived by cell wall receptors, and can alter immunity and cell wall composition. Therefore, we hypothesised that xylo-oligosaccharides (XOS) could act as an elicitor and trigger immune responses. To test this, we treated Arabidopsis with xylobiose (XB) and investigated different parameters. XB-treatment significantly triggered the generation of reactive oxygen species (ROS), activated MAPK protein phosphorylation, and induced callose deposition. The combination of XB (DAMP) and flg22 a microbe-associated molecular pattern (MAMP) further enhanced ROS response and gene expression of PTI marker genes. RNA sequencing analysis revealed that more genes were differentially regulated after 30 min compared to 24 h XB-treated leaves, which correlated with ROS response. Increased xylosidase activity and soluble xylose level after 30 min and 3 h of XB-treatment were observed which might have weakened the DAMP response. However, an increase in total cell wall sugar and a decrease in uronic acid level was observed at both 30 min and 24 h. Additionally, arabinose, rhamnose, and xylose levels were increased in 30 min, and glucose was increased in 24 h compared to mock-treated leaves. The level of jasmonic acid, abscisic acid, auxin, and cytokinin were also affected after XB treatment. Overall, our data revealed that the shortest XOS can act as a DAMP, which triggers the PTI response and alters cell wall composition and hormone level.
Hide AbstractLignocellulose Degrading Weizmannia coagulans Capable of Enantiomeric L-Lactic Acid Production via Consolidated Bioprocessing.
Pamueangmun, P., Abdullahi, A. D., Kabir, M. H., Unban, K., Kanpiengjai, A., Venus, J., Shetty, K., Saenjum, C. & Khanongnuch, C. (2023). Fermentation, 9(8), 761.
Second-generation lactic acid production requires the development of sustainable and economically feasible processes and renewable lignocellulose biomass as a starting raw material. Weizmannia coagulans MA42 was isolated from a soil sample in Chiang Mai province, Thailand and showed the highest production of L-lactic acid and lignocellulolytic enzymes (cellulase, β-mannanase, xylanase, β-glucosidase, β-mannosidase, and β-xylosidase) compared to other isolates. Weizmannia coagulans MA42 was able to grow, secrete lignocellulolytic enzymes, and directly produce L-lactic acid in the medium containing various lignocellulosic feedstocks as the sole carbon source. Moreover, L-lactic acid production efficiency was improved after the substrates were pretreated with diluted sulfuric acid and diluted sodium hydroxide. The highest L-lactic acid production efficiency of 553.4 ± 2.9, 325.4 ± 4.1, 326.6 ± 4.4, 528.0 ± 7.2, and 547.0 ± 2.2 mg/g total available carbohydrate was obtained from respective pretreated substrates including sugarcane bagasse, sugarcane trash, corn stover, rice straw, and water hyacinth. It is suggested that structural complexity of the lignocellulosic materials and properties of lignocellulolytic enzymes are the key factors of consolidated bioprocessing (CBP) of lignocellulosic feedstocks to lactic acid. In addition, the results of this study indicated that W. coagulans MA42 is a potent bacterial candidate for CBP of a variety of lignocellulosic feedstocks to L-lactic acid production; however, further bioprocess development and genetic engineering technique would provide higher lactic acid production efficiency, and this would lead to sustainable lactic acid production from lignocellulosic feedstocks.
Hide AbstractStructure and enzymatic characterization of CelD endoglucanase from the anaerobic fungus Piromyces finnis.
Dementiev, A., Lillington, S. P., Jin, S., Kim, Y., Jedrzejczak, R., Michalska, K., Joachimiak, A. & O’Malley, M. A. (2023). Applied Microbiology and Biotechnology, 107(19), 5999-6011.
Anaerobic fungi found in the guts of large herbivores are prolific biomass degraders whose genomes harbor a wealth of carbohydrate-active enzymes (CAZymes), of which only a handful are structurally or biochemically characterized. Here, we report the structure and kinetic rate parameters for a glycoside hydrolase (GH) family 5 subfamily 4 enzyme (CelD) from Piromyces finnis, a modular, cellulosome-incorporated endoglucanase that possesses three GH5 domains followed by two C-terminal fungal dockerin domains (double dockerin). We present the crystal structures of an apo wild-type CelD GH5 catalytic domain and its inactive E154A mutant in complex with cellotriose at 2.5 and 1.8 Å resolution, respectively, finding the CelD GH5 catalytic domain adopts the (β/α)8-barrel fold common to many GH5 enzymes. Structural superimposition of the apo wild-type structure with the E154A mutant-cellotriose complex supports a catalytic mechanism in which the E154 carboxylate side chain acts as an acid/base and E278 acts as a complementary nucleophile. Further analysis of the cellotriose binding pocket highlights a binding groove lined with conserved aromatic amino acids that when docked with larger cellulose oligomers is capable of binding seven glucose units and accommodating branched glucan substrates. Activity analyses confirm P. finnis CelD can hydrolyze mixed linkage glucan and xyloglucan, as well as carboxymethylcellulose (CMC). Measured kinetic parameters show the P. finnis CelD GH5 catalytic domain has CMC endoglucanase activity comparable to other fungal endoglucanases with kcat = 6.0 ± 0.6 s−1 and Km = 7.6 ± 2.1 g/L CMC. Enzyme kinetics were unperturbed by the addition or removal of the native C-terminal dockerin domains as well as the addition of a non-native N-terminal dockerin, suggesting strict modularity among the domains of CelD.
Hide AbstractArginine methyltransferases PRMT2 and PRMT3 are essential for biosynthesis of plant-polysaccharide-degrading enzymes in Penicillium oxalicum.
Zhao, S., Mo, L. X., Li, W. T., Jiang, L. L., Meng, Y. Y., Ou, J. F., Liao, L. S., Yan, Y. S., Luo, X. M. & Feng, J. X. (2023). PLoS Genetics, 19(7), e1010867.
Many filamentous fungi produce plant-polysaccharide-degrading enzymes (PPDE); however, the regulatory mechanism of this process is poorly understood. A Gal4-like transcription factor, CxrA, is essential for mycelial growth and PPDE production in Penicillium oxalicum. Its N-terminal region, CxrAΔ207–733 is required for the regulatory functions of whole CxrA, and contains a DNA-binding domain (CxrAΔ1–16&Δ59–733) and a methylated arginine (R) 94. Methylation of R94 is mediated by an arginine N-methyltransferase, PRMT2 and appears to induce dimerization of CxrAΔ1–60. Overexpression of prmt2 in P. oxalicum increases PPDE production by 41.4–95.1% during growth on Avicel, compared with the background strain Δku70;hphR+. Another arginine N-methyltransferase, PRMT3, appears to assist entry of CxrA into the nucleus, and interacts with CxrAΔ1–60 in vitro under Avicel induction. Deletion of prmt3 resulted in 67.0-149.7% enhanced PPDE production by P. oxalicum. These findings provide novel insights into the regulatory mechanism of fungal PPDE production.
Hide AbstractEssential autoproteolysis of bacterial anti-σ factor RsgI for transmembrane signal transduction.
Chen, C., Dong, S., Yu, Z., Qiao, Y., Li, J., Ding, X., LI, R., LIN, J., BAYER, E. A., LIU, Y. J., CUI, Q. & Feng, Y. (2023). Science Advances, 9(27), eadg4846.
Autoproteolysis has been discovered to play key roles in various biological processes, but functional autoproteolysis has been rarely reported for transmembrane signaling in prokaryotes. In this study, an autoproteolytic effect was discovered in the conserved periplasmic domain of anti-σ factor RsgIs from Clostridium thermocellum, which was found to transmit extracellular polysaccharide-sensing signals into cells for regulation of the cellulosome system, a polysaccharide-degrading multienzyme complex. Crystal and NMR structures of periplasmic domains from three RsgIs demonstrated that they are different from all known proteins that undergo autoproteolysis. The RsgI-based autocleavage site was located at a conserved Asn-Pro motif between the β1 and β2 strands in the periplasmic domain. This cleavage was demonstrated to be essential for subsequent regulated intramembrane proteolysis to activate the cognate SigI, in a manner similar to that of autoproteolysis-dependent activation of eukaryotic adhesion G protein-coupled receptors. These results indicate the presence of a unique prevalent type of autoproteolytic phenomenon in bacteria for signal transduction.
Hide AbstractNanohaloarchaea as beneficiaries of xylan degradation by haloarchaea.
La Cono, V., Messina, E., Reva, O., Smedile, F., La Spada, G., Crisafi, F., Marturano, L., Miguez, N., Ferrer, M., Selivanova, E. A., Golyshina, O. V., Golyshin, P. N., Rohde, M., Krupovic, M., Merkel, A. Y., Sorokin, D. Y., Hallsworth, J. E. & Yakimov, M. M. (2023). Microbial Biotechnology, 16(9), 1803-1822.
Climate change, desertification, salinisation of soils and the changing hydrology of the Earth are creating or modifying microbial habitats at all scales including the oceans, saline groundwaters and brine lakes. In environments that are saline or hypersaline, the biodegradation of recalcitrant plant and animal polysaccharides can be inhibited by salt-induced microbial stress and/or by limitation of the metabolic capabilities of halophilic microbes. We recently demonstrated that the chitinolytic haloarchaeon Halomicrobium can serve as the host for an ectosymbiont, nanohaloarchaeon 'Candidatus Nanohalobium constans'. Here, we consider whether nanohaloarchaea can benefit from the haloarchaea-mediated degradation of xylan, a major hemicellulose component of wood. Using samples of natural evaporitic brines and anthropogenic solar salterns, we describe genome-inferred trophic relations in two extremely halophilic xylan-degrading three-member consortia. We succeeded in genome assembly and closure for all members of both xylan-degrading cultures and elucidated the respective food chains within these consortia. We provide evidence that ectosymbiontic nanohaloarchaea is an active ecophysiological component of extremely halophilic xylan-degrading communities (although by proxy) in hypersaline environments. In each consortium, nanohaloarchaea occur as ectosymbionts of Haloferax, which in turn act as scavenger of oligosaccharides produced by xylan-hydrolysing Halorhabdus. We further obtained and characterised the nanohaloarchaea-host associations using microscopy, multi-omics and cultivation approaches. The current study also doubled culturable nanohaloarchaeal symbionts and demonstrated that these enigmatic nano-sized archaea can be readily isolated in binary co-cultures using an appropriate enrichment strategy. We discuss the implications of xylan degradation by halophiles in biotechnology and for the United Nation's Sustainable Development Goals.
Hide AbstractHorseradish peroxidase delignification of fermented rooibos modifies biomass structural and chemical properties and improves holocellulolytic enzyme cocktail efficacy.
Mohotloane, M. M., Alexander, O., Pletschke, B. I. & Mafa, M. S. (2023). Biologia, 78, 1943-1959.
The purpose of the study was to pretreat fermented rooibos biomass with partially purified horseradish peroxidase (HRP) for lignin removal and to convert delignified biomass to soluble sugars through saccharification with a formulated holocellulolytic enzyme cocktail (HEC). HRP enzyme was extracted from the horseradish root tissue and was partially purified by membrane filters and characterised biochemically. HRP enzyme was used to pretreat the fermented rooibos biomass to remove lignin before hydrolysing it with the HEC. Our findings indicated that HRP is versatile because it displayed activity on guaiacol, 8-aminoquinoline, and decolourised methylene blue dye. HRP had a pH optimum of 4.5 and displayed a mesophilic temperature range. The kinetics studies indicated that HRP displayed a higher affinity towards guaiacol (Km= 0.082 mg/mL) followed by 8-aminoquinoline (Km= 0.221 mg/mL). However, the catalytic efficiency revealed that the enzyme hydrolysed guaiacol (63436.48 s− 1. mg/mL) and 8-aminoquinoline (59189.81 s− 1. mg/mL) efficiently. HRP pretreatment of rooibos biomass significantly removed lignin content and increased pores on the surface as visualised with SEM. FTIR validated the SEM results by showing reductions at 3324.81, 1615.16 and 1018.75 cm− 1, corresponding to crystalline cellulose, lignin and holocellulose regions, respectively. HRP pretreated biomass had the lowest crystallinity index of 11.2% compared to 20% of the control. HRP delignified rooibos biomass was hydrolysed effectively by the HEC, which released about 10% yield of soluble sugars compared to 6% of control. We conclude that HRP pretreatment significantly modified the structural and chemical properties of the biomass, making it more accessible to hydrolytic enzymes.
Hide Abstract