2,000 Units
Prices exclude VAT
This product has been discontinued
| Content: | 2,000 Units |
| Shipping Temperature: | Ambient |
| Storage Temperature: | 2-8oC |
| Formulation: | In 3.2 M ammonium sulphate |
| Physical Form: | Suspension |
| Stability: | > 4 years at 4oC |
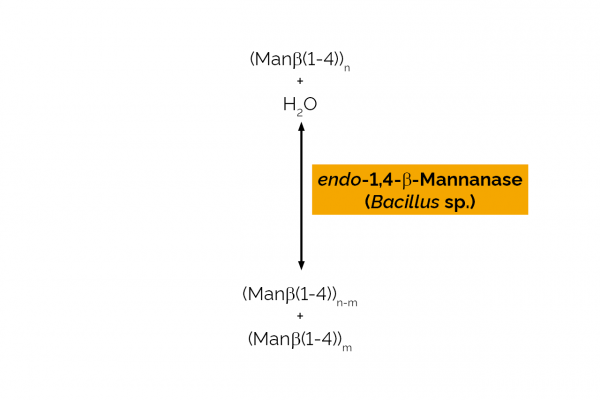
| Enzyme Activity: | endo-1,4-β-Mannanase |
| EC Number: | 3.2.1.78 |
| CAZy Family: | GH26 |
| CAS Number: | 37288-54-3 |
| Synonyms: | mannan endo-1,4-beta-mannosidase; 4-beta-D-mannan mannanohydrolase |
| Source: | Bacillus sp. |
| Molecular Weight: | 30,500 |
| Concentration: | Supplied at ~ 1,200 U/mL |
| Expression: | Purified from Bacillus sp. |
| Specificity: | Random hydrolysis of (1,4)-β-D-mannosidic linkages in mannans, galactomannans and glucomannans. |
| Specific Activity: | > 50 U/mg (40oC, pH 8.8 on carob galactomannan) |
| Unit Definition: | One Unit of β-mannanase activity is the amount of enzyme which releases one µmole of mannose-reducing-sugar equivalents per minute from low viscosity carob galactomannan (10 mg/mL) in glycine buffer (100 mM), pH 8.8 at 40oC. |
| Temperature Optima: | 65oC |
| pH Optima: | 8.8 |
| Application examples: | Applications established in diagnostics and research within the food and feed, carbohydrate, biofuels and paper production industries. |
This product has been discontinued (read more).
High purity endo-1,4 β-Mannanase (Bacillus sp.) for use in research, biochemical enzyme assays and in vitro diagnostic analysis.
View more related CAZyme products.
Measurement of α-amylase activity in white wheat flour, milled malt, and microbial enzyme preparations, using the ceralpha assay: Collaborative study.
McCleary, B. V., McNally, M., Monaghan, D. & Mugford, D. C. (2002). Journal of AOAC International, 85(5), 1096-1102.
This study was conducted to evaluate the method performance of a rapid procedure for the measurement of α-amylase activity in flours and microbial enzyme preparations. Samples were milled (if necessary) to pass a 0.5 mm sieve and then extracted with a buffer/salt solution, and the extracts were clarified and diluted. Aliquots of diluted extract (containing α-amylase) were incubated with substrate mixture under defined conditions of pH, temperature, and time. The substrate used was nonreducing end-blocked p-nitrophenyl maltoheptaoside (BPNPG7) in the presence of excess quantities of thermostable α-glucosidase. The blocking group in BPNPG7 prevents hydrolysis of this substrate by exo-acting enzymes such as amyloglucosidase, α-glucosidase, and β-amylase. When the substrate is cleaved by endo-acting α-amylase, the nitrophenyl oligosaccharide is immediately and completely hydrolyzed to p-nitrophenol and free glucose by the excess quantities of α-glucosidase present in the substrate mixture. The reaction is terminated, and the phenolate color developed by the addition of an alkaline solution is measured at 400 nm. Amylase activity is expressed in terms of Ceralpha units; 1 unit is defined as the amount of enzyme required to release 1 µmol p-nitrophenyl (in the presence of excess quantities of α-glucosidase) in 1 min at 40°C. In the present study, 15 laboratories analyzed 16 samples as blind duplicates. The analyzed samples were white wheat flour, white wheat flour to which fungal α-amylase had been added, milled malt, and fungal and bacterial enzyme preparations. Repeatability relative standard deviations ranged from 1.4 to 14.4%, and reproducibility relative standard deviations ranged from 5.0 to 16.7%.
Hide AbstractMallett, I., McCleary, B. V. & Matheson, N. K. (1987). Phytochemistry, 26(7), 1889-1894.
Galactomannan has been extracted from the endosperm of seeds of Gleditsia triacanthos (honey locust) at different stages of development, when the seed was accumulating storage material. Properties of the different samples have been studied. The molecular size distribution became more disperse as galactomannan accumulated and the galactose: mannose ratio decreased slightly. Some possible reasons for these changes are discussed.
Hide AbstractMeasurement of cereal α-Amylase: A new assay procedure.
McCleary, B. V. & Sheehan, H. (1987). Journal of Cereal Science, 6(3), 237-251.
A new procedure for the assay of cereal α-amylase has been developed. The substrate is a defined maltosaccharide with an α-linked nitrophenyl group at the reducing end of the chain, and a chemical blocking group at the non-reducing end. The substrate is completely resistant to attack by β-amylase, glucoamylase and α-glucosidase and thus forms the basis of a highly specific assay for α-amylase. The reaction mixture is composed of the substrate plus excess quantities of α-glucosidase and glucoamylase. Nitrophenyl-maltosaccharides released on action of α-amylase are instantaneously cleaved to glucose plus free p-nitrophenol by the glucoamylase and α-glucosidase, such that the rate of release of p-nitrophenol directly correlates with α-amylase activity. The assay procedure shows an excellent correlation with the Farrand, the Falling Number and the Phadebas α-amylase assay procedures.
Hide AbstractDea, I. C. M., Clark, A. H. & McCleary, B. V. (1986). Carbohydrate Research, 147(2), 275-294.
A range of galactomannans varying widely in the contents of D-galactose have been compared for self-association and their interaction properties with agarose and xanthan. Whereas, in general, the most interactive galactomannans are those in which the (1→4)-β-D-mannan chain is least substituted by α-D-galactosyl stubs, evidence is presented which indicates that the distribution of D-galactosyl groups along the backbone (fine structure) can have a significant effect on the interaction properties. For galactomannans containing <30% of D-galactose, those which contain a higher frequency of unsubstituted blocks of intermediate length in the β-D-mannan chain are most interactive. For galactomannans containing >40% of D-galactose, those which contain a higher frequency of exactly alternating regions in the β-D-mannan chain are most interactive. This selectivity, on the basis of galactomannan fine-structure, in mixed polysaccharide interactions in vitro could mimic the selectivity of binding of branched plant-cell-wall polysaccharides in biological systems.
Hide AbstractEffect of the molecular fine structure of galactomannans on their interaction properties - the role of unsubstituted sides.
Dea, I. C. M., Clark, A. H. & McCleary, B. V. (1986). Food Hydrocolloids, 1(2), 129-140.
A range of galactomannans varying widely in the content of D-galactose have been compared for self-association, and their interaction properties with agarose and xanthan. The results presented indicate that in general the most interactive galactomannans are those in which the D-mannan main chain bears fewest D-galactose stubs, and confirm that the distribution of D-galactose groups along the main chain can have a significant effect on the interactive properties of the galactomannans. It has been shown that freeze — thaw precipitation of galactomannans requires regions of totally unsubstituted D-mannose residues along the main chain, and that a threshold for significant freeze — thaw precipitation occurs at a weight-average length of totally unsubstituted residues of approximately six. For galactomannans having structures above this threshold their interactive properties with other polysaccharides are controlled by structural features associated with totally unsubstituted regions of the D-mannan backbone. In contrast, for galactomannans below this threshold, their interactive properties are controlled by structural features associated with unsubstituted sides of D-mannan backbone.
McCleary, B. V., Clark, A. H., Dea, I. C. M. & Rees, D. A. (1985). Carbohydrate Research, 139, 237-260.
The distribution of D-galactosyl groups along the D-mannan backbone (fine structure) of carob and guar galactomannans has been studied by a computer analysis of the amounts and structures of oligosaccharides released on hydrolysis of the polymers with two highly purified β-D-mannanases isolated from germinated guar seed and from Aspergillus niger cultures. Computer programmes were developed which accounted for the specific subsite-binding requirements of the β-D-mannanases and which simulated the synthesis of galactomannan by processes in which the D-galactosyl groups were transferred to the growing D-mannan chain in either a statistically random manner or as influenced by nearest-neighbour/second-nearest-neighbour substitution. Such a model was chosen as it is consistent with the known pattern of synthesis of similar polysaccharides, for example, xyloglucan; also, addition to a preformed mannan chain would be unlikely, due to the insoluble nature of such polymers. The D-galactose distribution in carob galactomannan and in the hot- and cold-water-soluble fractions of carob galactomannan has been shown to be non-regular, with a high proportion of substituted couplets, lesser amounts of triplets, and an absence of blocks of substitution. The probability of sequences in which alternate D-mannosyl residues are substituted is low. The probability distribution of block sizes for unsubstituted D-mannosyl residues indicates that there is a higher proportion of blocks of intermediate size than would be present in a galactomannan with a statistically random D-galactose distribution. Based on the almost identical patterns of amounts of oligosaccharides produced on hydrolysis with β-D-mannanase, it appears that galactomannans from seed of a wide range of carob varities have the same fine-structure. The D-galactose distribution in guar-seed galactomannan also appears to be non-regular, and galactomannans from different guar-seed varieties appear to have the same fine-structure.
Hide AbstractMcCleary, B. V. (1983). Carbohydrate Research, 111(2), 297-310.
β-D-Mannosidase (β-D-mannoside mannohydrolase EC 3.2.1.25) was purified 160-fold from crude gut-solution of Helix pomatia by three chromatographic steps and then gave a single protein band (mol. wt. 94,000) on SDS-gel electrophoresis, and three protein bands (of almost identical isoelectric points) on thin-layer iso-electric focusing. Each of these protein bands had enzyme activity. The specific activity of the purified enzyme on p-nitrophenyl β-D-mannopyranoside was 1694 nkat/mg at 40° and it was devoid of α-D-mannosidase, β-D-galactosidase, 2-acet-amido-2-deoxy-D-glucosidase, (1→4)-β-D-mannanase, and (1→4)-β-D-glucanase activities, almost devoid of α-D-galactosidase activity, and contaminated with <0.02% of β-D-glucosidase activity. The purified enzyme had the same Km for borohydride-reduced β-D-manno-oligosaccharides of d.p. 3-5 (12.5mM). The initial rate of hydrolysis of (1→4)-linked β-D-manno-oligosaccharides of d.p. 2-5 and of reduced β-D-manno-oligosaccharides of d.p. 3-5 was the same, and o-nitrophenyl, methylumbelliferyl, and naphthyl β-D-mannopyranosides were readily hydrolysed. β-D-Mannobiose was hydrolysed at a rate ~25 times that of 61-α-D-galactosyl-β-D-mannobiose and 63-α-D-galactosyl-β-D-mannotetraose, and at ~90 times the rate for β-D-mannobi-itol.
Hide AbstractMcCleary, B. V. (1983). Phytochemistry, 22(3), 649-658.
Hydrolysis of galactomannan in endosperms of germinating guar is due to the combined action of three enzymes, α-galactosidase, β-mannanase and exo-β-mannanase. α-Galactosidase and exo-β-mannanase activities occur both in endosperm and cotyledon tissue but β-mannanase occurs only in endosperms. On seed germination, β-mannanase and endospermic α-galactosidase are synthesized and activity changes parallel galactomannan degradation. Galactomannan degradation and synthesis of these two enzymes are inhibited by cycloheximide. In contrast, endospermic exo-β-mannanase is not synthesized on seed germination, but rather is already present throughout endosperm tissue. It has no action on native galactomannan. α-Galactosidase, β-mannanase and exo-β-mannanase have been purified to homogeneity and their separate and combined action in the hydrolysis of galactomannan and effect on the rate of uptake of carbohydrate by cotyledons, studied. Results obtained indicated that these three activities are sufficient to account for galactomannan degradation in vivo and, further, that all three are required. Cotyledons contain an active exo-β-mannanase and sugar-uptake experiments have shown that cotyledons can absorb mannobiose intact, indicating that this enzyme is involved in the complete degradation of galactomannan on seed germination.
Hide AbstractMcCleary, B. V., Nurthen, E., Taravel, F. R. & Joseleau, J. P. (1983). Carbohydrate Research, 118, 91-109.
Treatment of hot-water-soluble carob galactomannan with β-D-mannanases from A. niger or lucerne seed affords an array of D-galactose-containing β-D-mannosaccharides as well as β-D-manno-biose, -triose, and -tetraose (lucerne-seed enzyme only). The D-galactose-containing β-D-mannosaccharides of d.p. 3–9 produced by A. niger β-D-mannanase have been characterised, using enzymic, n.m.r., and chemical techniques, as 61-α-D-galactosyl-β-D-mannobiose, 61-α-D-galactosyl-β-D-mannotriose, 63,64-di-α-D-galactosyl-β-D-mannopentaose (the only heptasaccharide), and 63,64-di-α-D-galactosyl-β-D-mannohexaose, 64,65-di-α-D-galactosyl-β-D-mannohexaose, and 61, 63,64-tri-α-D-galactosyl-β-D-mannopentaose (the only octasaccharides). Four nonasaccharides have also been characterised. Penta- and hexa-saccharides were absent. Lucerne-seed β-D-mannanase produced the same branched tri-, tetra- and hepta-saccharides, and also penta- and hexa-saccharides that were characterised as 61-α-D-galactosyl-β-D-mannotetraose, 63-α-D-galactosyl-β-D-mannotetraose, 61,63-di-α-D-galactosyl-β-D-mannotetraose, 63-α-D-galactosyl-β-D-mannopentaose, and 64-α-D-galactosyl-β-D-mannopentaose. None of the oligosaccharides contained a D-galactose stub on the terminal D-mannosyl group nor were they substituted on the second D-mannosyl residue from the reducing terminal.
Hide AbstractMcCleary, B. V. & Matheson, N. K. (1983). Carbohydrate Research, 119, 191-219.
Purified (1→4)-β-D-mannanase from Aspergillus niger and lucerne seeds has been incubated with mannosaccharides and end-reduced (1→4)-β-D-mannosaccharides and, from the products of hydrolysis, a cyclic reaction-sequence has been proposed. From the heterosaccharides released by hydrolysis of the hot-water-soluble fraction of carob galactomannan by A. niger β-D-mannanase, a pattern of binding between the β-D-mannan chain and the enzyme has been deduced. The products of hydrolysis with the β-D-mannanases from Irpex lacteus, Helix pomatia, Bacillus subtilis, and lucerne and guar seeds have also been determined, and the differences from the action of A. niger β-D-mannanase related to minor differences in substrate binding. The products of hydrolysis of glucomannan are consistent with those expected from the binding pattern proposed from the hydrolysis of galactomannan.
Hide AbstractMcCleary, B. V. (1982), Carbohydrate Research, 101(1), 75-92.
A β-D-mannoside mannohydrolase enzyme has been purified to homogeneity from germinated guar-seeds. Difficulties associated with the extraction and purification appeared to be due to an interaction of the enzyme with other protein material. The purified enzyme hydrolysed various natural and synthetic substrates, including β-D-manno-oligosaccharides and reduced β-D-manno-oligosaccharides of degree of polymerisation 2 to 6, as well as p-nitrophenyl, naphthyl, and methylumbelliferyl β-D-mannopyranosides. The preferred, natural substrate was β-D-mannopentaose, which was hydrolysed at twice the rate of β-D-mannotetraose and five times the rate of β-D-mannotriose. This result, together with the observation that α-D-mannose is released on hydrolysis, indicates that the enzyme is an exo-β-D-mannanase.
Hide AbstractMcCleary, B. V., Taravel, F. R. & Cheetham, N. W. H. (1982). Carbohydrate Research, 104(2), 285-297.
N.m.r., enzymic, and chemical techniques have been used to characterise the D-galactose-containing tri- and tetra-saccharides produced on hydrolysis of carob and L. leucocephala D-galacto-D-mannans by Driselase β-D-mannanase. These oligosaccharides were shown to be exclusively 61-α-D-galactosyl-β-D-mannobiose and 61-α-D-galactosyl-β-D-mannotriose. Furthermore, these were the only D-galactose-containing tri- and tetra-saccharides produced on hydrolysis of carob D-galacto-D-mannan by β-D-mannanases from other sources, including Bacillus subtilis, Aspergillus niger, Helix pomatia gut solution, and germinated legumes. Acid hydrolysis of lucerne galactomannan yielded 61-α-D-galactosyl-β-D-mannobiose and 62-α-D-galactosyl-β-D-mannobiose.
Hide AbstractAn enzymic technique for the quantitation of galactomannan in guar Seeds.
McCleary, B. V. (1981). Lebensmittel-Wissenschaft & Technologie, 14, 56-59.
An enzymic technique has been developed for the rapid and accurate quantitation of the galactomannan content of guar seeds and milling fractions. The technique involves the measurement of the galactose component of galactomannans using galactose dehydrogenase. The galactomannans are converted to galactose and manno-oligosaccharides using partially purified enzymes from a commercial preparation and from germinated guar seeds. Simple procedures have been devised for the preparation of these enzymes. Application of the technique to a number of guar varieties gave values for the galactomannan content ranging from 22.7 to 30.8% of seed weight.
Hide AbstractMcCleary, B. V. (1979). Phytochemistry, 18(5), 757-763.
β-Mannanase activities in the commercial enzyme preparations Driselase and Cellulase, in culture solutions of Bacillus subtilis (TX1), in commercial snail gut (Helix pomatia) preparations and in germinated seeds of lucerne, Leucaena leucocephala and honey locust, have been purified by substrate affinity chromatography on glucomannan-AH-Sepharose. On isoelectric focusing, multiple protein bands were found, all of which had β-mannanase activity. Each preparation appeared as a single major band on SDS-polyacrylamide gel electrophoresis. The enzymes varied in their final specific activities, Km values, optimal pH, isoelectric points and pH and temperature stabilities but had similar MWs. The enzymes have different abilities to hydrolyse galactomannans which are highly substituted with galactose. The preparations Driselase and Cellulase contain β-mannanases which can attack highly substituted galactomannans at points of single unsubstituted D-mannosyl residues if the D-galactose residues in the vicinity of the bond to be hydrolysed are all on only one side of the main chain.
Hide AbstractMcCleary, B. V., Matheson, N. K. & Small, D. B. (1976). Phytochemistry, 15(7), 1111-1117.
A series of galactomannans with varying degrees of galactose substitution have been extracted from the endosperms of legume seeds with water and alkali and the amount of substitution required for water solubility has been determined. Some were heterogeneous with respect to the degree of galactose substitution. The structural requirements for hydrolysis by plant β-mannanase have been studied using the relative rates and extents of hydrolysis of these galactomannans. A more detailed examination of the products of hydrolysis of carob galactomannan has been made. At least two contiguous anhydromannose units appear to be needed for scission. This is similar to the requirement for hydrolysis by microbial enzymes. Judas tree (Cercis siliquastrum) endosperm contained a polysaccharide with a unique composition for a legume seed reserve. Gel chromatography and electrophoresis on cellulose acetate indicated homogeneity. Hydrolysis with a mixture of β-mannanase and α-galactosidase gave a glucose-mannose disaccharide and acetolysis gave a galactose-mannose. These results, as well as the pattern of hydrolysis by β-mannanase were consistent with a galactoglucomannan structure.
Hide AbstractMcCleary, B. V. & Matheson, N. K. (1975). Phytochemistry, 14(5-6), 1187-1194.
Structural changes in galactomannan on germination of lucerne, carob, honey locust, guar and soybean seeds, as measured by viscosity, elution volumes on gel filtration and ultra-centrifugation were slight consistent with a rapid and complete hydrolysis of a molecule once hydrolysis of the mannan chain starts. β-Mannanase activity increased and then decreased, paralleling galactomannan depletion. Multiple forms of β-mannanase were isolated and these were located in the endosperm. β-Mannanase had limited ability to hydrolyse galactomannans with high galactose contents. Seeds containing these galactomannans had very active α-galactosidases. β-Mannosidases were present in both endosperm and cotyledon-embryo and could be separated chromatographically. The level of activity was just sufficient to account for mannose production from manno-oligosaccharides.
Hide Abstract